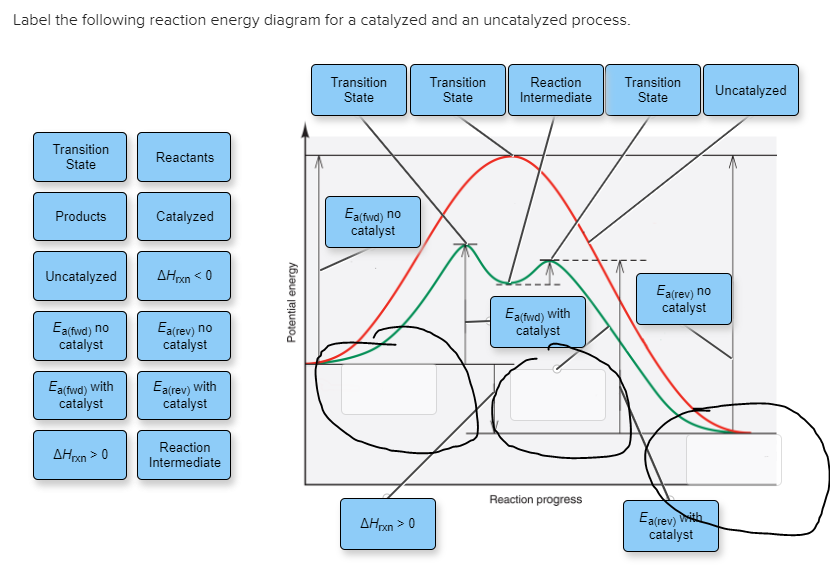
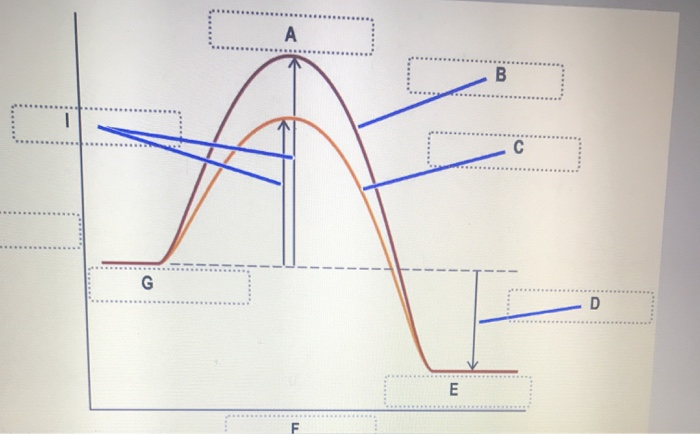
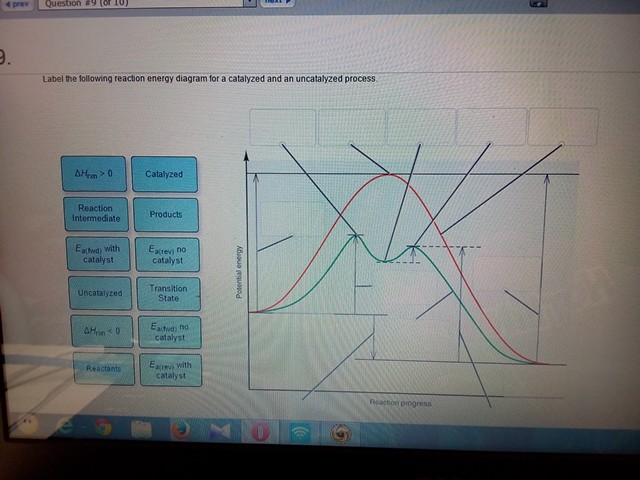
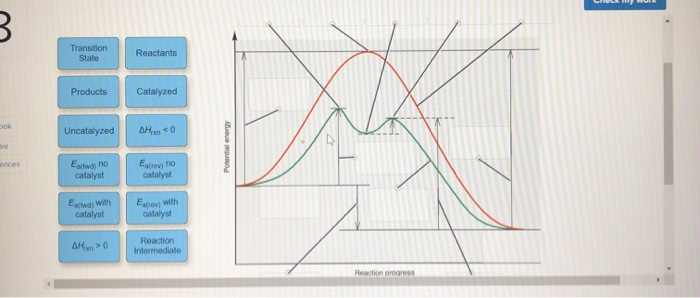
44 label the following reaction energy diagram for a catalyzed and an uncatalyzed process
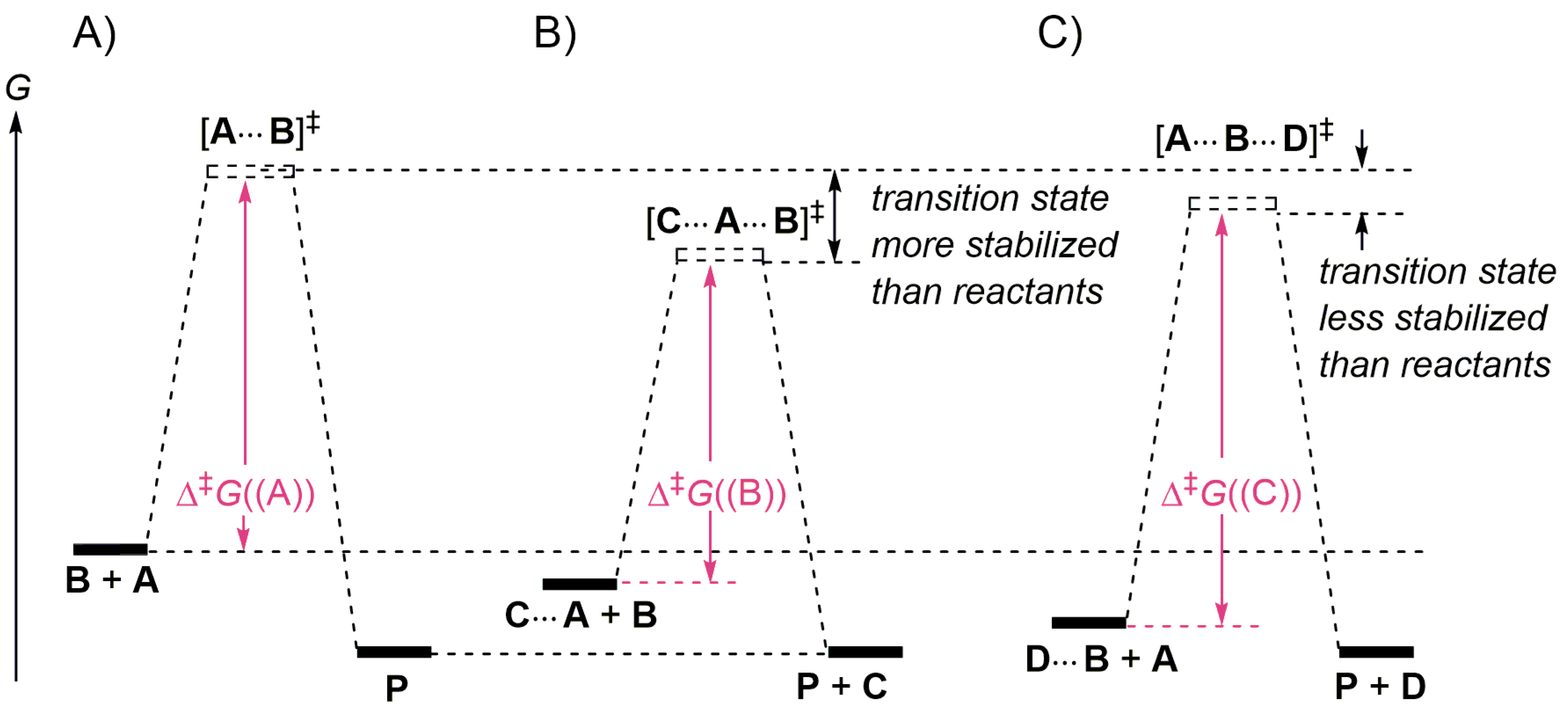
PDF AP CHEMISTRY 2006 SCORING GUIDELINES - College Board (d) Consider the four reaction-energy profile diagrams shown below. (i) Identify the two diagrams that could represent a catalyzed and an uncatalyzed reaction pathway for the same reaction. Indicate which of the two diagrams represents the catalyzed reaction pathway for the reaction. Diagram 1 represents a catalyzed pathway and diagram 2 Reaction profiles - Exothermic and endothermic reactions - BBC The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions. The diagram shows a reaction profile for an exothermic reaction.
Solved Label the following reaction energy diagram for a - Chegg Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. A > 0 Eatwa) no catalyst Exter, with catalyst Reactants Esinev) no Eastw with catalyst Potential energy Mono Products Uncatalyzed Catalyzed Transition State Reaction Intermediate Reaction progress Reset Zoom This problem has been solved! See the answer
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process
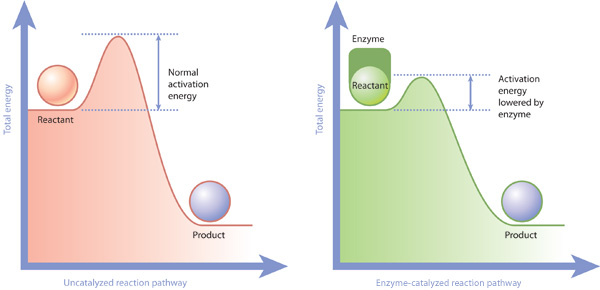
[Solved] Suzuki Post-Lab 1. Safety Question Define PEL, TLV, and flash ... For a catalyzed process assume that the rate determining step is the transmetallation of the complex analogous to Complex A to produce the complex analogous to Complex B in PowerPoint slide 18 Label the following in your potential energy diagram: i. Reactants ii. Product iii. AH* for the uncatalyzed process and value given. iv . 12.7 Catalysis | General College Chemistry II - Lumen Learning One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to afford the saturated alkane product. A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. Figure 1. Catalysis | Chemistry for Majors - Lumen Learning Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. The catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two transitions states).
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. A catalyst speeds up a chemical reaction by lowering the activation ... Label the two traces as "uncatalyzed" and "catalyzed." A catalyst speeds up a chemical reaction by lowering the activation energy. Sketch Diagram A and draw a second line on the same diagram for the reaction if a catalyst is present. Label the two traces as "uncatalyzed" and "catalyzed." Textbook Question Chapter 5, Problem 1IA.3Q 7.8 - Catalysis - General Chemistry for Gee-Gees Answer. Diagram (b) is a catalyzed reaction with an activation energy of about 70 kJ. Homogeneous Catalysts. A homogeneous catalyst is present in the same phase as the reactants. It interacts with a reactant to form an intermediate substance, which then decomposes or reacts with another reactant in one or more steps to regenerate the original catalyst and form product. Label the following reaction energy diagram for a catalyzed and an ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. posted on April 23, 2022 what is the difference between catalyzed and uncatalyzed reactions A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. d. In the presence of a catalyst at 37°C, the rate constant for the reaction increases by a factor of 2.50 103 as compared with the uncatalyzed reaction.
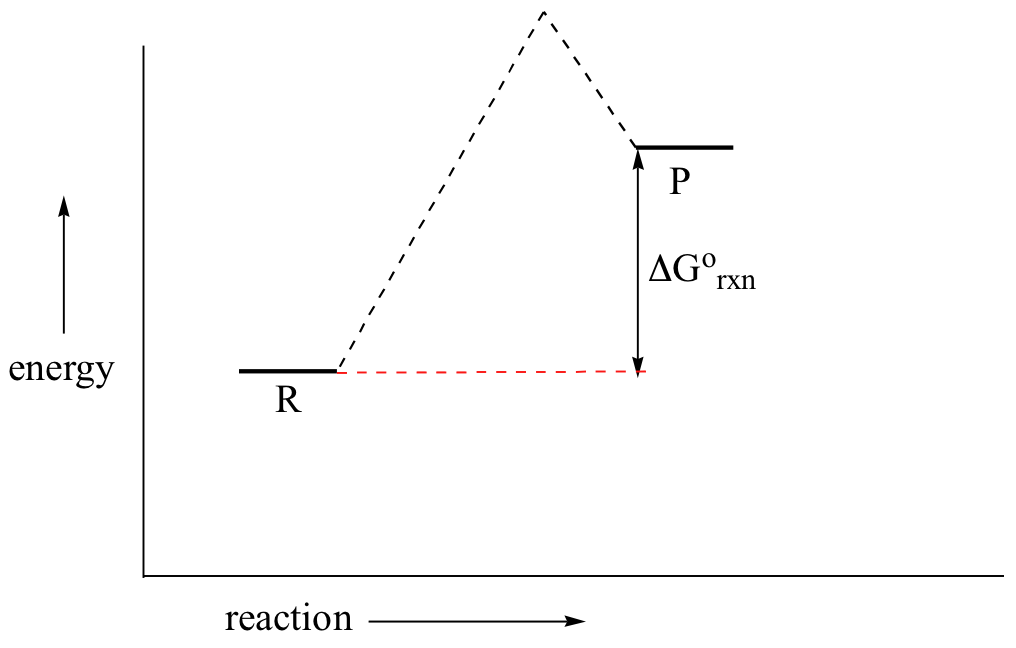
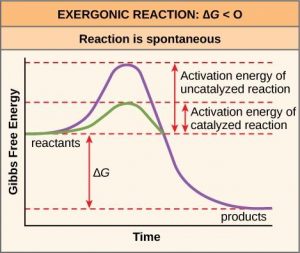
Chapter 8 BIOCHEM Flashcards | Quizlet Label the energy reaction graph for the following reaction showing the energy profile for a catalyzed and an uncatalyzed reaction. Ea, rxn not cat by enzyme, rxn cat by enzyme, energy released by rxn A.Look at the graph of reaction rate versus substrate concentration for an enzyme.In which region does the reaction rate remain constant? B. Label the following reaction energy diagram for a catalyzed and an ... SOMEONE ASKED 👇. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. PDF Graph Review Wed - North St. Paul-Maplewood Oakdale / Overview Below is an energy diagram for a particular process. One curve represents the energy profile for the uncatalyzed reaction, and the other curve represents the energy profile for the catalyzed reaction. Course reaction a. N%ich curve has the geater activation energy? Curve 1 or cunre 2? b. How to draw the potential energy diagram for this reaction? C3H8(g) + 5O2(g) → 3CO2(g) +4H2O(g) + 2219.9 kJ, we say that ΔH ∘ C = − 2219.9 kJ/mol propane. We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction.
PDF Energy/Reaction Coordinate Diagrams - chemconnections A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change ... Bonds are NOT in the process of breaking or forming. ! Energy Diagrams # Intermediates! 6! For each of the diagrams below, will the transition state ... Label each of those atoms nucleophilic (nu) or electrophilic (el) in each resonance ... Solved Label the following reaction energy diagram for a - Chegg Expert Answer 100% (66 ratings) All the labeling you have done are correct except Hrxn < 0. Hrxn<0 because i … View the full answer Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Enzymes are important molecules in biochemistry that - SolutionInn Enzymes are important molecules in biochemistry that catalyze reactions. The energy diagram illustrates the difference between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram. PDF ap07 chemistry q6 - College Board The reaction is exothermic. The reaction is slow at 25°C; however, a catalyst will cause the reaction to proceed faster. (e) Using the axes provided below, draw the complete potential-energy diagram for both the catalyzed and uncatalyzed reactions. Clearly label the curve that represents the catalyzed reaction.
PDF Chemical kinetics Name: Date - The Leon M. Goldstein High School for ... On the diagram below, draw a potential energy diagram for this reaction. 18. Base your answer(s) to the following question(s) on the reaction represented by the balanced equation below. 2H2(g)+O2(g) !2H2O(')+571:6kJ On the axes below, draw a potential energy diagram for the reaction represented by this equation. 19. Given the reaction at ...
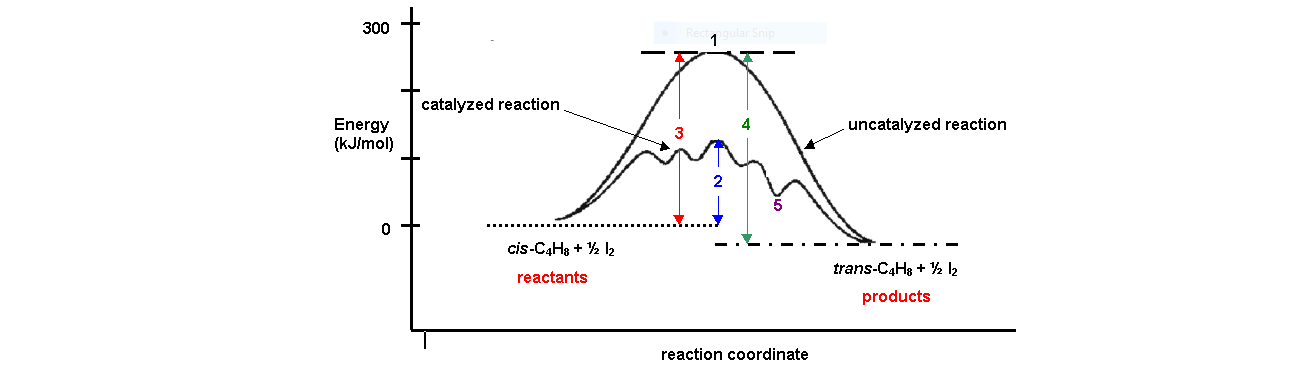
The graph that best describes catalyzed and uncatalyzed exothermic ... In the presence of a palladium catalyst, the ethyne forms ethene and ethane. This reaction is represented by the unbalanced equation C2H2(g) + H2(g) C2H4(g) + C2H6(g) + energy The energy diagram that represents both the catalyzed (---) and uncatalyzed reactions (___) is a. c. b. d. Upload your study docs or become a
Label the reactants and products on the enthalpy diagrams for each process. Q: At 552.3 k, the rate constant for the thermal decomposition of f so2cl2 is 1.02k2*10^-6. If the activation energy is 210kj/mol determine the rate constant at 700k. Posted 26 days ago. Q: CHM 703 Electro-analytical Techniques 1a. Explain the principle of anodic stripping voltammetry. Why is stripping the most sensitive polarographic technique?
PDF Potential Energy Diagram Worksheet ANSWERS A catalyst changes the reaction mechanism, in the process lowering the activation energy. 5. Name 4 things that will speed up or slow down a chemical reaction. Increase concentration by distillation of a solvent, Increase concentration by increasing pressure of a gas, Increase temp, Add a catalyst, Add an inhibitor. 6. Draw an energy diagram ...
(Solved) - Question: Consider The Reaction Profile Diagram For The ... QUESTIONS: 1. Use The Reaction ...
Biochemistry Mastering Ch. 8 Post-lecture Flashcards | Quizlet The enzyme urease catalyzes the hydrolysis of urea to ammonia plus carbon dioxide. At 28 ∘C the uncatalyzed reaction has an activation energy of about 123 kJ/mol, whereas in the presence of urease the activation energy is lowered to about 44 kJ/mol. By what factor does urease increase the velocity of the reaction?
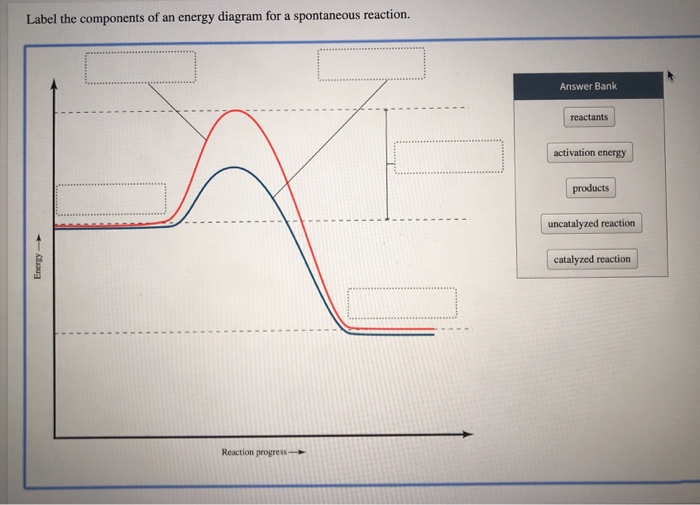
Answered: Label the components of an energy… | bartleby Q: Label the energy diagram (7 bins) and indicate which reaction corresponds to the energy diagram.… A: The solution is provided in the next step: Q: Which of the following is NOT a reasonable assumption about the chemical reaction whose energy… A: Given answer of choices, The activation energy for the reaction is 100 kJ The reaction is… Q: B
PDF The Leon M. Goldstein High School for the Sciences The potential energy diagram below represents a reaction. Reaction Coordínate Which arrow represents the activation energy of the forward reaction? (1) A 2) B 3) C (4) D 5. Which phase changeis anexothermic process? (2) NH3 (g) (1) cu(s) cu(l) (4) Hg(l) Hg(g) 16. Sketch the potential energy diagram for an endothermic chemical reaction that ...
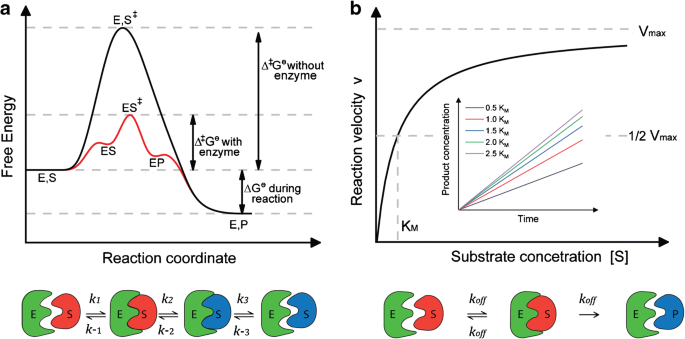
Energy diagrams for enzyme‐catalyzed reactions: Concepts and ... - IUBMB The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. The survey of several Biochemistry textbooks reveals a high diversity of profiles for the same process. A, C, and D are adapted from Refs. 5, 3, and 2, respectively.
Catalysis | Chemistry for Majors - Lumen Learning Reaction diagrams for an endothermic process in the absence (red curve) and presence (blue curve) of a catalyst. The catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two transitions states).
12.7 Catalysis | General College Chemistry II - Lumen Learning One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to afford the saturated alkane product. A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. Figure 1.
[Solved] Suzuki Post-Lab 1. Safety Question Define PEL, TLV, and flash ... For a catalyzed process assume that the rate determining step is the transmetallation of the complex analogous to Complex A to produce the complex analogous to Complex B in PowerPoint slide 18 Label the following in your potential energy diagram: i. Reactants ii. Product iii. AH* for the uncatalyzed process and value given. iv .





















Post a Comment for "44 label the following reaction energy diagram for a catalyzed and an uncatalyzed process"