43 open-label trial advantages and disadvantages
Open-Label Extension Studies | SpringerLink The number of open-label extension studies being performed has increased enormously in recent years. Often it is difficult to differentiate between these extension studies and the double-blind, controlled studies that preceded them. If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label ... Open-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 An open-label trial of escitalopram for SAD patients ( N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of …
Confidentiality in Peer Review: Single Blind, Double Blind, Open One of the advantages of a Double blind process is the academic objectivity it insures despite, for example, the author's stature in a particular research community. In a survey of 3,000 academics, 71% said they have confidence in the double blind peer review process.2 This fact alone is a positive.
Open-label trial advantages and disadvantages
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology trial were eligible to participate in the open-label portion. During this randomized phase, there were 32/104 patients randomized to placebo that dropped out before completing 24 weeks of treatment, but 23 who did not take part in the open-label extension. There were 8/101 patients randomized to etanercept who dropped out, but 14 who did not ... Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for ... Attitudes towards open‐label versus placebo‐control designs in ... Feb 21, 2022 ... However, open-label design can be associated with several limitations such as possible higher patient dropout, concerns regarding the internal ...
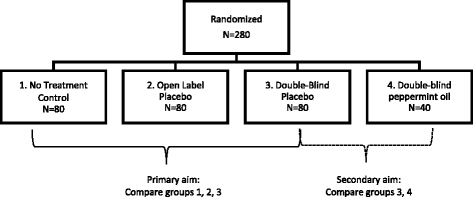
Open-label trial advantages and disadvantages. Reducing bias in open-label trials where blinded outcome assessment is ... This can be an issue in trials assessing surgical interventions, device trials, or other non-pharmacologic interventions, which are more difficult to blind than traditional drug trials [ 4 ]. Many such trials are therefore open-label, where patients, clinicians, and care providers are aware of treatment allocations. Self-Manage Scleroderma | Lesson Patients have a chance to help others and improve patient care. Some disadvantages might be: New treatments or interventions under study are not always better than, or even as good as, standard care. Even if a new treatment has benefits, it may not work for everyone. Health insurance and managed care providers don't always cover clinical trials. Guidance for Clinical Trial Sponsors - Food and Drug Administration highlight these advantages and disadvantages, with particular attention to the setting in which investigational products are being evaluated for possible marketing approval in well-controlled ... External and internal validity of open label or double-blind trials in ... ... 22 However, open-label trials offer the advantage of enhanced generalizability because the treatment quality in the warfarin control group may be more representative of routine clinical...
Safety and Immunogenicity of a DNA SARS-CoV-2 ... - eClinicalMedicine We conducted a single-center, open-label, non-randomized, Phase 1 trial in India between July 2020 and October 2020. Healthy adults aged between 18 and 55 years were sequentially enrolled and allocated to one of four treatment arms in a dose escalation manner. ... Vaccine administration using NFIS device offers some distinct advantages compared ... Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized. Open Clinical Trials | Bioclever Blog Dec 10, 2020 ... Open Clinical Trials · It's easier to conduct a non-blinded study than a blind study. · Investigators may feel more comfortable to make decisions ... Full article: Effectiveness studies: advantages and disadvantages Apr 1, 2022 ... Lower study costs ; Studies with an active control, Supply data on relative efficacy and tolerability, Risk of false studies because assay ...
Open-Label Trial | NIH - HIV.gov Open-Label Trial. A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Designs of clinical trials - SlideShare Trial with Zelen's design... Advantages: 1. Almost all eligible individuals are included in the trial. 2. Allows the evaluation of true effect of experimental intervention in patients. Disadvantages: 1. Open trials. 2. The statistical power of the study gets compromised if large no of patients choose the standard treatment. 2/18/2015 67 68. Crossover trials: what are they and what are their advantages and ... Crossover trials can only be conducted when the disease persists for a longer period of time, hence, crossover trials are mostly used in studying chronic diseases. There are some short-term illnesses or acute conditions that might be cured once they are treated and there are treatments that will have a permanent effect (i.e. surgery) on the ... Open-Label Trial - an overview | ScienceDirect Topics Open-label trials of desipramine, tranylcypromine, reboxetine, and bupropion showed improvement with the drug therapy.55 An open-label trial of escitalopram for SAD patients ( N = 20) over 8 weeks produced a response rate of 95% (SIGH-SAD < 50% of baseline value) and a remission rate (SIGH-SAD score < 8) of 85%. 56 In an open-label trial of …
External and internal validity of open label or double-blind trials in ... In these trials, open-label or double-blind double-dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients.
Exploration and Ethical Analysis of Open-label Pediatric Vaccine Trials ... One option for gathering data on tolerability and efficacy in children would be to use a nonrandomized trial to enroll parents willing to vaccinate their children and those who are hesitant. We discuss the advantages and disadvantages of such an open-label trial that could provide guidance for future pandemics. (Clin Ther.
What is an Open-Label Clinical Trial? - News-Medical.net Several benefits for open-label trials have been suggested by researchers, including the ongoing access to medicine by phase III pivotal trial volunteers which have been proven effective, but...
Treatment Use of Investigational Drugs | FDA There are four requirements that must be met before a treatment IND can be issued: 1) the drug is intended to treat a serious or immediately life-threatening disease; 2) there is no satisfactory...
14 Product Trial Advantages and Disadvantages - BrandonGaille.com List of the Advantages of a Product Trial 1. It gives a product the chance to sell itself. When you have a great product, then it can serve as its own marketing tool. Great products sell themselves. Customers who get to see this fact during their free product trial will see this for themselves.
14 Advantages and Disadvantages of a Randomized Controlled Trial - Vittana List of the Advantages of Randomized Controlled Trials 1. Randomization prevents the deliberate manipulation of results. A randomized controlled trial works to prevent skewing or the deliberate manipulation of results by researchers or participants.
External and internal validity of open label or double‐blind trials in ... In these trials, open‐label or double‐blind double‐dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients.
RCT & Adaptive clinical trial designs | Credevo Articles Advantages of RCT. RCTs are the most reliable form of scientific evidence in the hierarchy of evidence. Randomized controlled trials reduce spurious causality and bias. The RCTs results may be combined in systematic reviews and are increasingly in use in the conduct of evidence-based practice. Disadvantages. RCTs can be expensive.
Epidemiology and Clinical Research Design, Part 1: Study Types. Strengths and limitations of retrospective studies: The main advantages of studies using existing data are speed and cost. A retrospective analysis of an association between caffeine and necrotizing enterocolitis (NEC) can be conducted in a few months using a neonatal intensive care unit (NICU) database with minimal expense. ( 6) Strengths:
Advantages and disadvantages of clinical trials Possible advantages You may have access to new treatments, which may only be available as part of a clinical trial. There may be fewer side effects compared to the standard treatment. You may have more regular tests, which some people find reassuring. You will be supported by a research nurse - who you can contact about your treatment and symptoms.
External and internal validity of open label or double‐blind trials in ... As a consequence, an intense discussion of the advantages and disadvantages of both designs is currently under way and interpretation of trial results is often focused on this matter. This review addresses the risks of bias for internal and external validity of open-label and double-blind anticoagulation trials to help to objectify this debate.
Understanding Clinical Trial Terminology: What is an Open Label ... Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ...
Cluster-randomised controlled trial - Wikipedia Disadvantages compared with individually randomised controlled trials include greater complexity in design and analysis, and a requirement for more participants to obtain the same statistical power. [2] Use of this type of trial also means that the experiences of individuals within the same group are likely similar, leading to correlated results.
What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities.
(PDF) What is an open label trial? - ResearchGate The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to...
Attitudes towards open‐label versus placebo‐control designs in ... Feb 21, 2022 ... However, open-label design can be associated with several limitations such as possible higher patient dropout, concerns regarding the internal ...
Open-label extension studies: do they provide meaningful ... - PubMed Negative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for ...
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology trial were eligible to participate in the open-label portion. During this randomized phase, there were 32/104 patients randomized to placebo that dropped out before completing 24 weeks of treatment, but 23 who did not take part in the open-label extension. There were 8/101 patients randomized to etanercept who dropped out, but 14 who did not ...
































Post a Comment for "43 open-label trial advantages and disadvantages"