39 open-label study bias
Original Article Bias was reduced in an open-label trial through the ... A recent review found that only 26% of open-label trials reported using blinded outcome assessment [20]. Unblinded outcome assessment can lead to biased ... Patient-Reported Outcomes: Open-Label Trials Can Be Designed ... Jul 22, 2022 ...
Open-label trial - Wikipedia An open-label trial may still be randomized. Open-label trials may also be uncontrolled (without a placebo group), with all participants receiving the same ...
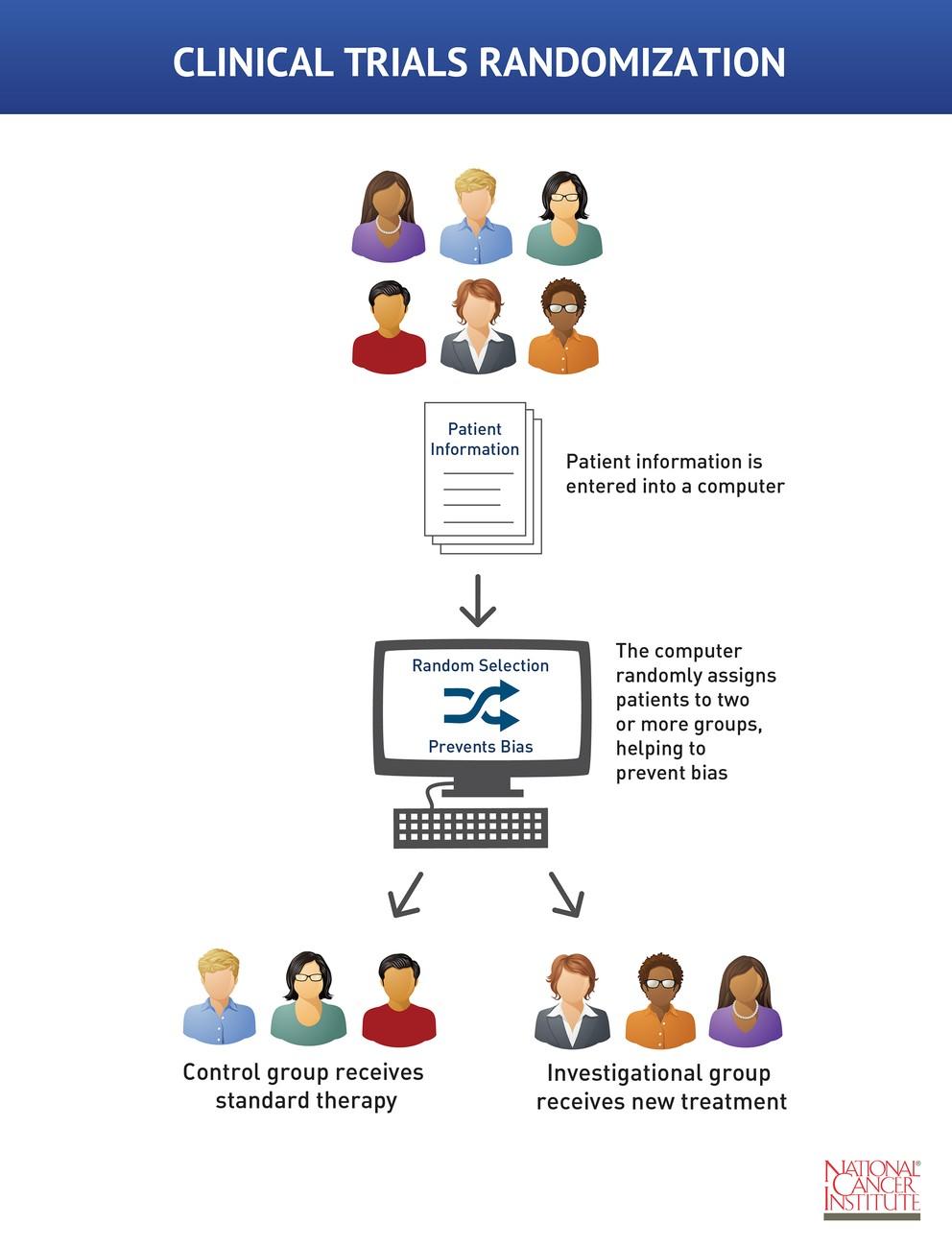
Open-label study bias
Investigating Potential Bias in Patient-Reported Outcomes in Open ... Jan 17, 2019 ... For example, one concern about open-label bias is that patients assigned to the investigational arm may be overly optimistic regarding the ... Assessing the impact of open-label designs in patient-reported ... The European Medicines Agency recommends blinded designs for PRO measurement in oncology studies to avoid concerns about bias in open-label settings, however, ... What is an Open-Label Clinical Trial? - News Medical Mar 31, 2022 ... Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled, this data is not of ...
Open-label study bias. Reducing bias in open-label trials where blinded outcome ... Nov 21, 2014 ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators ... 8.4 Introduction to sources of bias in clinical trials A useful classification of biases is into selection bias, performance bias, attrition bias, detection bias and reporting bias. In this section we describe each ... Investigating the impact of open label design on patient‐reported ... Aug 26, 2020 ... Our findings suggest that there is no evidence of significant bias for PROs due to the absence of blinding in the context of PCa RCTs. Further ... Bias for Patient-Reported Outcomes in Open-Label Cancer Trials Feb 27, 2019 ... One such bias is that patients on the investigational arm may be “overly optimistic,” whereas patients on the control arm may be “overly ...
What is an Open-Label Clinical Trial? - News Medical Mar 31, 2022 ... Open-label trials can increase the confidence about incidence rates, but as they are typically biased and uncontrolled, this data is not of ... Assessing the impact of open-label designs in patient-reported ... The European Medicines Agency recommends blinded designs for PRO measurement in oncology studies to avoid concerns about bias in open-label settings, however, ... Investigating Potential Bias in Patient-Reported Outcomes in Open ... Jan 17, 2019 ... For example, one concern about open-label bias is that patients assigned to the investigational arm may be overly optimistic regarding the ...







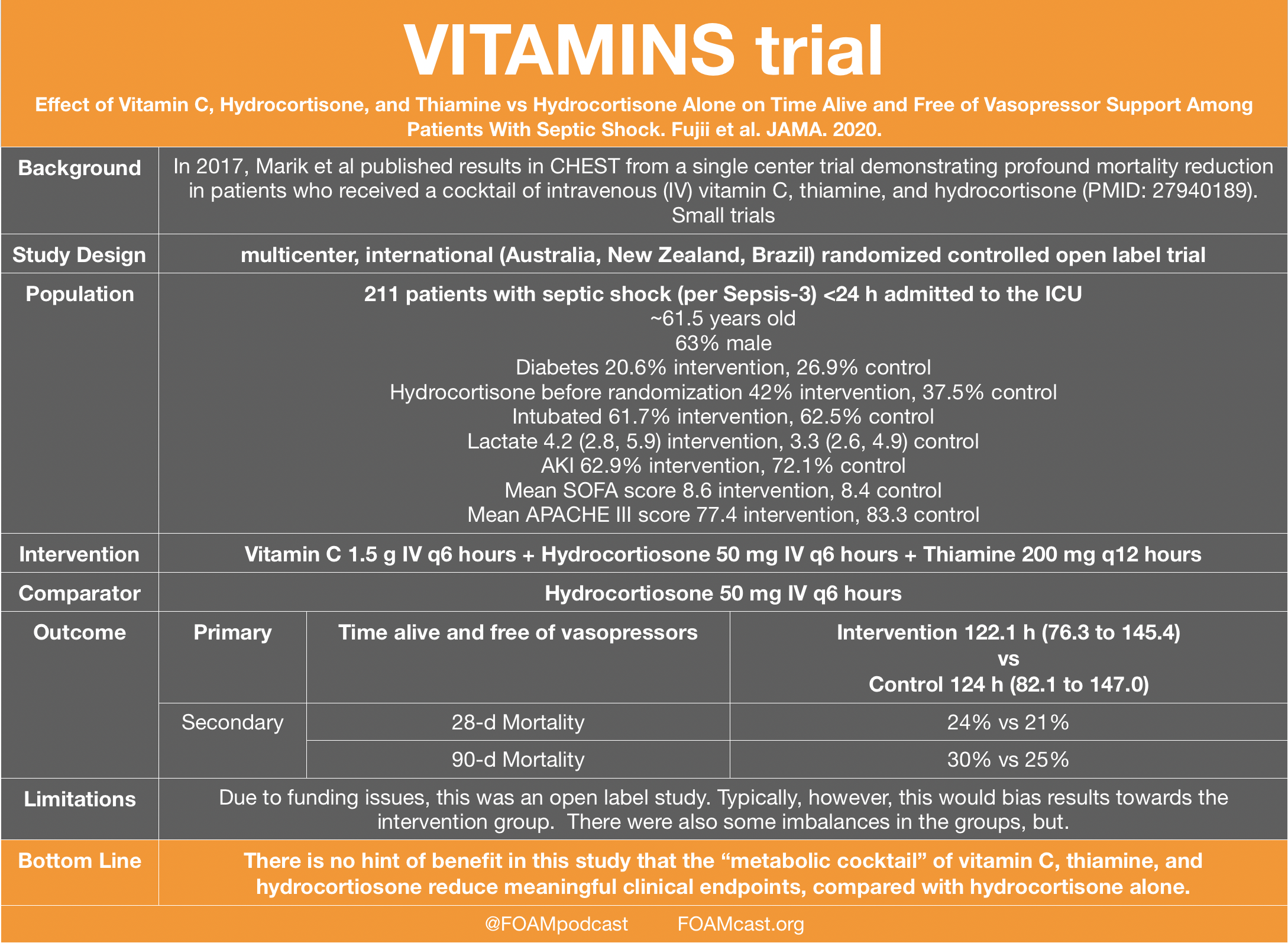



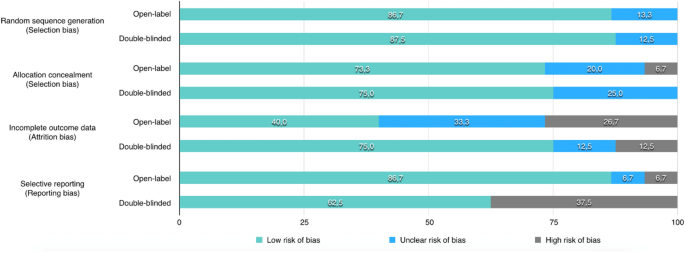











![PDF] Clinical trial methodology to assess the efficacy ...](https://d3i71xaburhd42.cloudfront.net/a00f5af9933a8f9c61da678199905782adf4210d/8-Table4-1.png)

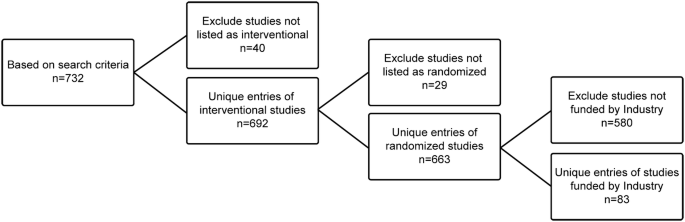



Post a Comment for "39 open-label study bias"